Design Process
Project Overview


This project involved creating a water filter to filter out sediment from contaminated water. We determined how "clean" the water was based on turbidity (measured in Nephelometric Turbidity Units, NTUs) instead of bacterial growth.
Define a Problem
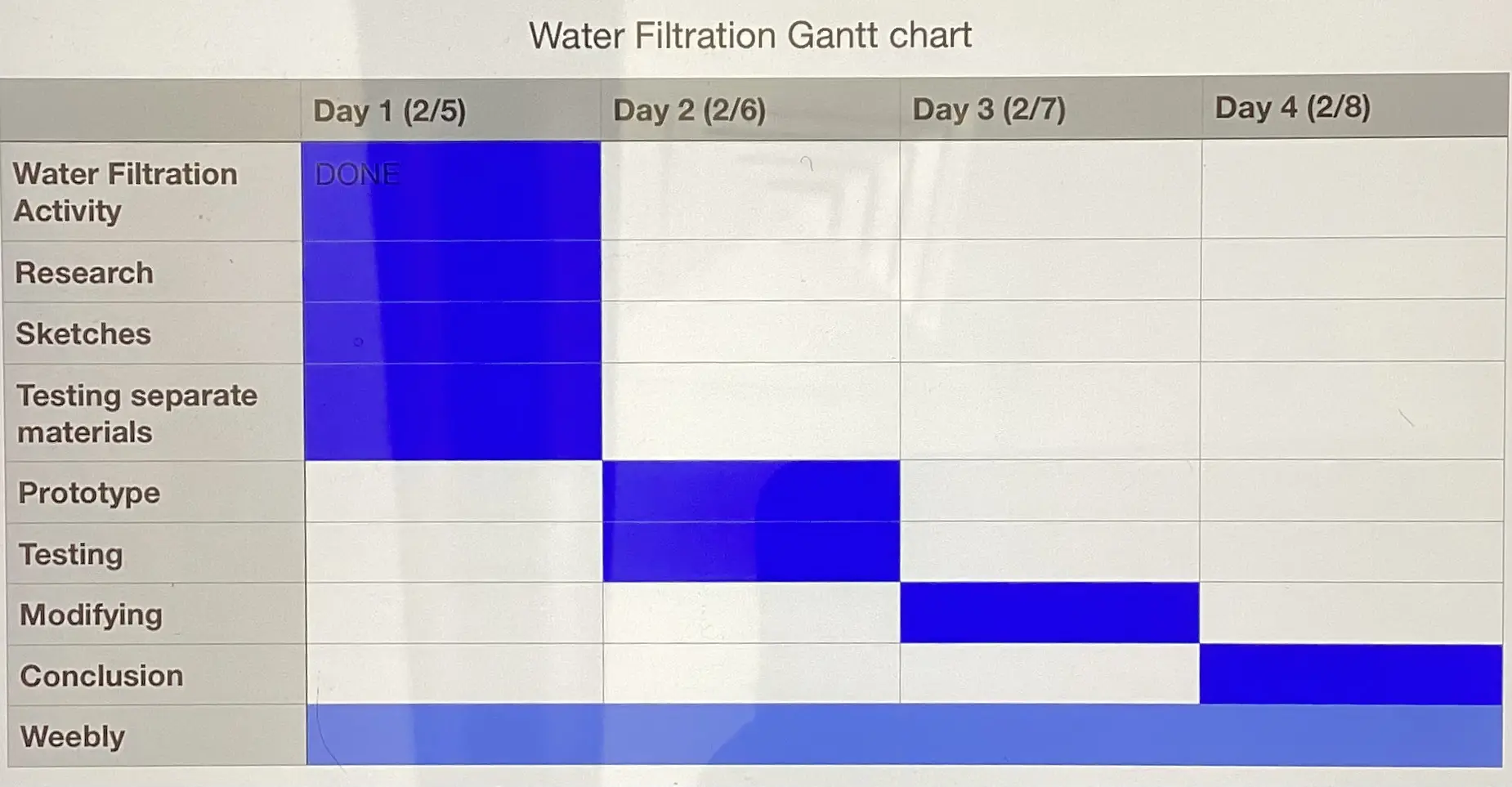
A Gantt Chart (First Picture) is very helpful in planning a timeline for any project. This helps to ensure that deadlines are met and that time is used effectively. Team responsibilities are also vital to assign in order to ensure effective and productive teamwork. This allows each team member to contribute their strengths, thus increasing the quality of the end result.

Generate Concepts
We brainstormed multiple materials to be used in the design:
* Cotton balls
* Coffee filters
* Paper towels
* Hankerchiefs
* Sand
* Gravel
* Charcoal
* Tissues
* Wipes
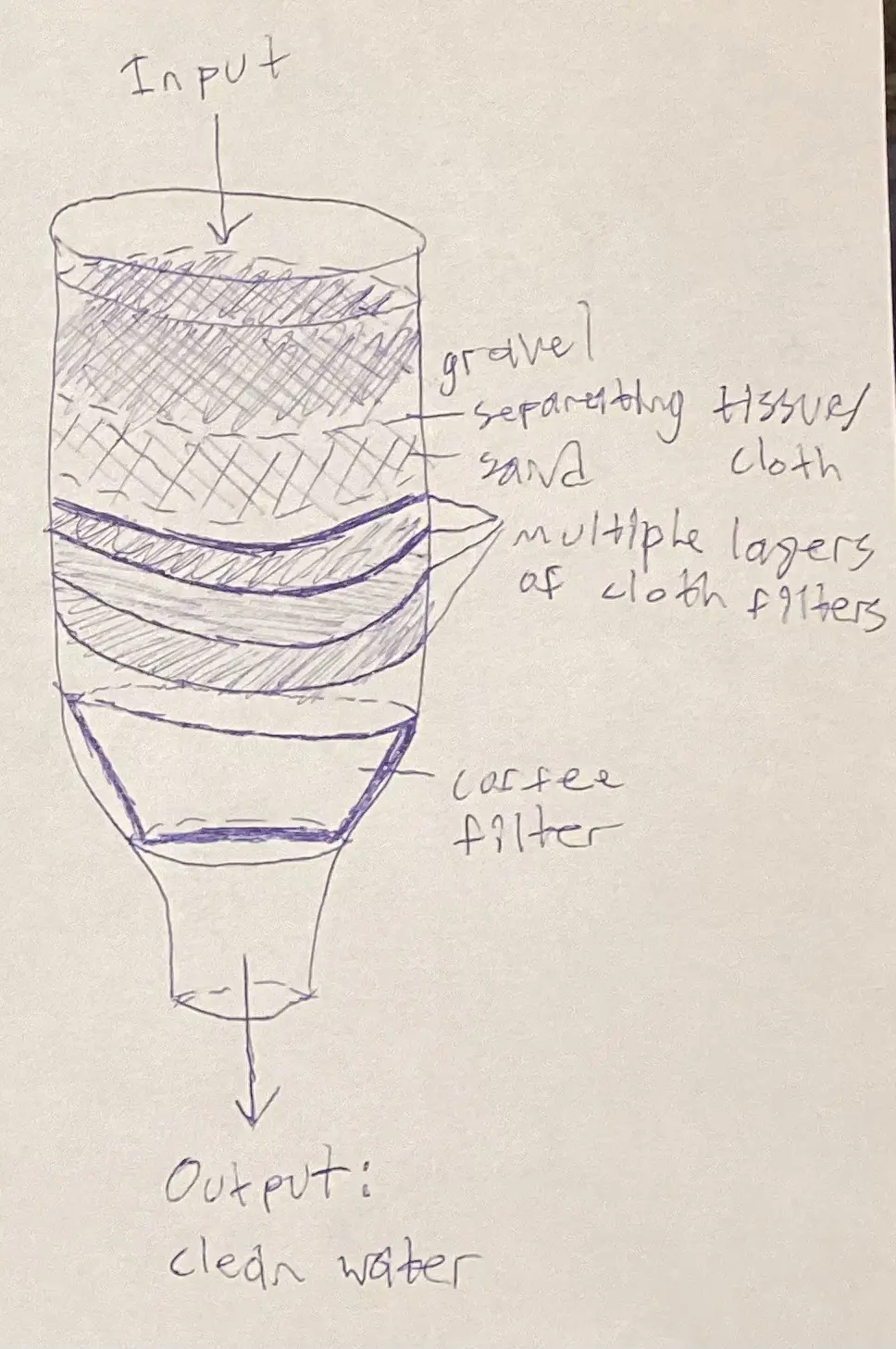
Previous Research
Bell Museum Water Bottle Filter

This water filter from the Bell Museum from the University of Minnesota uses household materials such as coffee filters, cotton balls, sand, and small rocks. However, it also uses charcoal, which we later found out to increase turbidity (while it actually kills bacteria, but that is not the goal of this project).
Pros:
* Simple design (Aligns with Occam's Razor)
* Can be made from household materials (mostly)
* Uses natural materials to filter water (sand, rocks), similar to how water is filtered in a forest ecosystem
Cons:
* Uses charcoal (increases turbidity but decreases bacterial growth)
* Sand may be contaminated, must be washed beforehand for low NTUs.
Source: Available Here

Utah State University Water Filter
Some professors at Utah State University created a homemade water filter design to educate students on the topic of filtration and disinfection.
Pros:
* Has multiple material suggestions, such as gravel, fine napkins and sand
* Focus on pathogen reduction, which is not a goal of this filter project but is still informative
Cons:
* Uses activated charcoal for pathogen reduction which unfortunately increases turbidity
* Does not use coffee filters or finer materials to filter out more sediment.
* These filter recommendations did not include any test results for turbidity.
Source: Available Here

MIT Xylem Water Filter
A team at MIT developed a water filter using treated pieces of wood.
First the wood was dipped in 60 degree Celsius water.
Then it was placed into cold water. I believe this was done to soften the wood.
Next, the wood sample was placed into ethanol, possibly to sterilize the wood itself.
It was then dried, likely to remove any residual ethanol.
The theory behind this water filter is that trees use negative pressure in their xylem to transport water from their roots to their leaves. The issue with this system is that vapor bubbles can form in the xylem which can increase the pressure, resulting in the water transport being disrupted. To solve this issue and prevent vapor bubbles from forming, a tree xylem has many pores that trap the vapor bubbles to prevent them from increasing in size.
Pros:
* Extremely effective water filtering, drinkable output!
* Uses only a single piece of treated wood to filter water effectively!
* These water filters are usable multiple times and still deliver drinkable water!
Cons:
* Requires treatment of wood, which we are unable to do at this time.
* The wood filters water so well that immense pressure (provided through a small tube and gravity) is required to force the water through the wood filter.
Source: Available Here
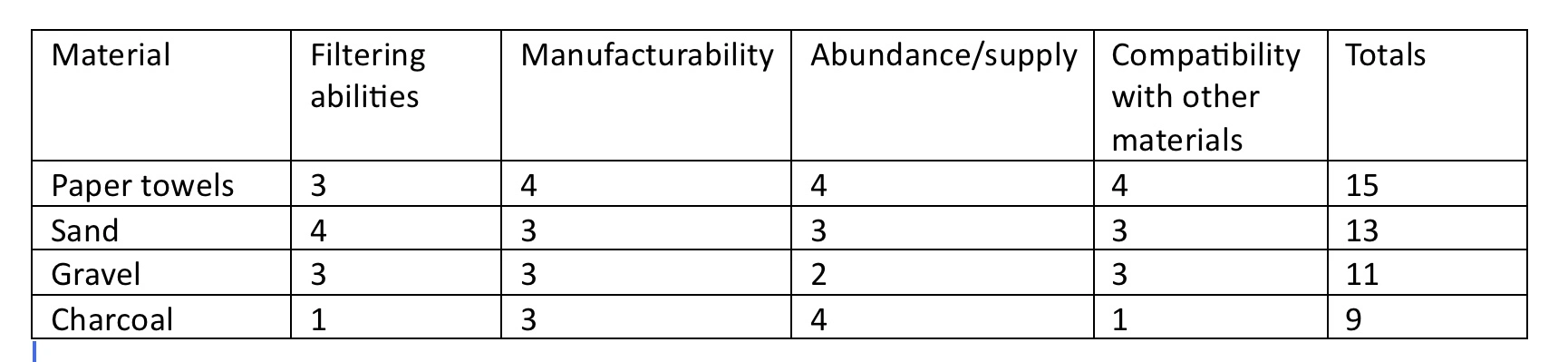
Research: Material Testing Results
In order to determine the most effective filter materials, we tested a variety of materials individually for accurate measurement. Aquatic substrate (course sand) filtered quite well as small particles were sifted out during filtering. The issue with using sand for filtering was that some sand could leak through the coffee filter at the bottom, causing an NTU reading of 100 NTUs. Aquarium gravel filtered based on a similar principle to that of sand and was unable to pass through the filter itself, resulting in a reading of 80 NTUs. While some materials were forced through the coffee filter, other materials such as thicker cloth and KN-95 masks were inpenetrable by water, even after being soaked for multiple minutes. Even a single layer cut from the mask was unpassible by water. This is likely the case due to the fact that masks are designed to filter out particles from air, which has much smaller particles than water droplets. The cloth may have been too thick or could be synthetic and waterproofed. A thicker paper towel resulted in a turbidity reading of 135 NTUs, but multiple layers of thinner paper towels proved to be extremely effective. We also tested charcoal, but the goal of this project is only to improve water clarity by minimizing turbidity, and charcoal is used only to remove bacteria. In fact, the charcoal resulted in a turbidity of 400 NTUs, as it mixed with the water and formed a homogenous mixture. It is possible that we used too much charcoal, did not filter out the charcoal itself, or did not apply the charcoal in its correct state while in our filter.
Decision Matrix: (Key: 1=worst, 5=Best)
Develop a Solution
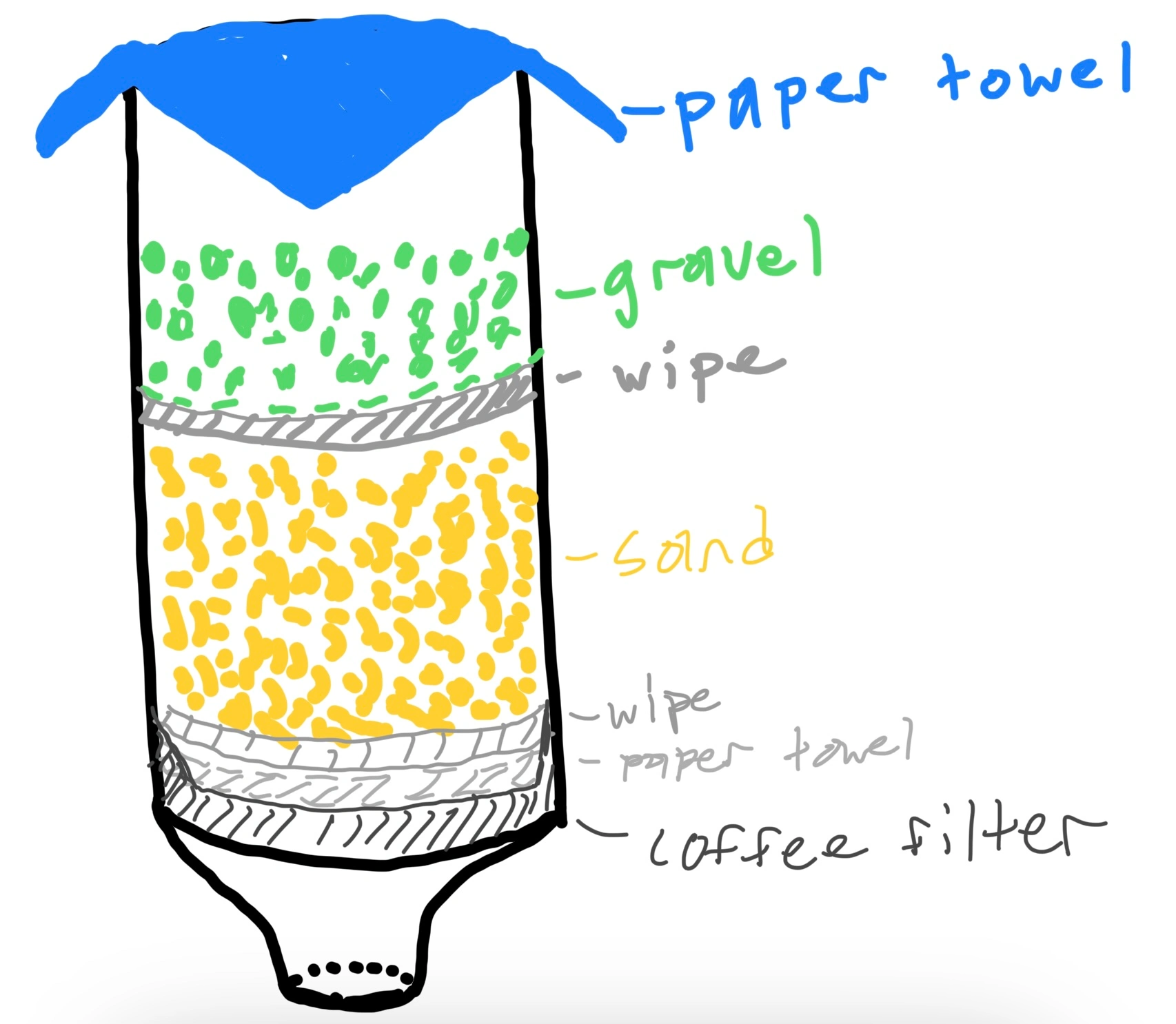
Construct and Test Prototype



The Effect of Various Materials on Turbidity
| Material | Turbidity (NTUs) |
| First Iteration - Coffee filter, wipes, sand, gravel, coarse tissues | 256.3 |
| Second Iteration - Added cotton balls to separate layers along with multiple fine tissues | 137.2 |
| After Running Clean Water Through Filter | 3.4 - Incorrect Due to Clean Water Stored in Filter |
| Final Design - Added more tissues in "DIY coffee filter funnel" form, also in alternating arrangements to ensure the mixture was adequately filtered. Forgot to clean sand and gravel beforehand. | 11.1 |
Overall, we needed to test multiple designs and iterations of our design in order to obtain an acceptable turbidity. Increasing the amount of layers proved to greatly reduce the turbidity. Along with this change, separating layers using cotton balls was also imperative. We also noticed that sand could spill out of the paper towels and bypass a few layers, causing an increase in turbidity of the result sample. To mitigate this issue, we decided to create "DIY coffee filters" by folding a paper towel first in half, and then into a triangle that could be opened up in a funnel/cone-like form. This allowed the water and sand to be directed through each layer of thin paper towels. This technique resulted in a substantial decrease in turbidity from 256.3 NTUs to 137.2 NTUs and later (after rinsing with clean water and recreating the filter using new materials) from 137.2 NTUs to 11.1 NTUs.
Evaluate Solution
As visible in the table above (in Construct and Test Prototype), we went through multiple iterations of our design. Each time, we improved the design by increasing the amount of layers, rearranging layers from coarse at the top to fine at the bottom, and by creating funnels to reduce the probability of a potential bypass or overflow in any given layer.
Present Solution
Together, Logan and I presented our final water filter design along with test results of materials, observations, and our collective design process. The presentation was given to our class where teams presented observations along with their final design to other teams. The presentation along with this portfolio serve as documentation how to successfully design, construct, and test a filter to reduce water turbidity using only household materials.
Reflection
This project was very informative and allowed me to explore the theoretical and practical aspects of water filters. Logan and I observed that a water filter could be improved by increasing the amount of layers along with starting with filtering out large particles with a hankerchief, gravel, and coarse paper towels. The water was then funneled through finer materials such as sand, thin paper towels, a coffee filter, and cotton to catch any last sedimentary particles. This was possible due to productive and fun teamwork. To further improve the project, I would test more samples and even try reusing the filter after cleaning materials, although this would likely take more time.
Our final turbidity was reduced from ~300NTUs to only 11.1 NTUs.